We’ve all seen the headlines: recent drug shortages have been the worst on record.1 This reality is problematic for patients, providers and manufacturers. At Fresenius Kabi, our purpose is to help patients gain better access to the life-saving treatments they need – when and where they need them most. To that end, we are constantly doing more in America to expand and enhance our production and supply chain network. But we realize that solving the problem of drug shortages will require both individual and collective actions. Unwinding the conditions that contribute to shortages will require new ways of thinking and collaborating. It will also require that each participant in the supply chain of care take steps to improve their own resilience.
With that in mind, we’ve been taking bold steps to build our resilience right here in the U.S.
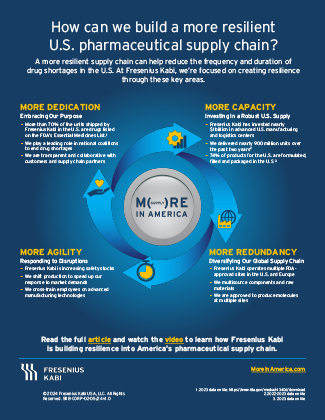
Years ago, we saw the need to commit to U.S. pharmaceutical manufacturing to help address the industrywide history of recurring shortages. As a result, Fresenius Kabi has invested nearly $1 billion to significantly expand our U.S. manufacturing and distribution capabilities. We are bringing a new, custom-built facility online in North Carolina for the manufacture of our innovative freeflex® IV solutions portfolio; in addition, we’ve transformed our New York and Illinois plants with advanced production technology – all to help us maintain best-in-class manufacturing standards to assure consistency and reliability for today’s essential medications. More capacity means that today more than 70% of all our products for the U.S. are formulated, filled and packaged in the U.S.2
To be better prepared for whatever comes next, our blueprint for resiliency embraces four drivers of performance3:
- MORE Capacity – As a century old global company, we believe in building capabilities “in country for country.” That’s why we are leading the way in onshoring and growing a local American supply of essential medicines and infusion technology. With the launch of our North Carolina facility, our plan is to more than double our U.S. manufacturing capacity in the next 5 years. Add to that, our three U.S. plants and three distribution centers are further backed by our expansive FDA-approved global manufacturing network.
See how Fresenius Kabi is creating more manufacturing resilience in our 2024 Infographic
[View PDF]
- MORE Redundancy – One of the best ways we can assure that the unexpected doesn’t disrupt our manufacturing is by having a diversified supply chain of reliable, reputable suppliers. Over the last few years, we have qualified numerous secondary sources for critical APIs and components in addition to qualifying the same product at different plants within our network, giving us the flexibility to increase capacity if needed. In addition, we standardize much of our cutting-edge technology and processes across our manufacturing lines so that people can move seamlessly from one machine or location to another when needed. Fresenius Kabi has a team of over 40,000 people worldwide dedicated to helping solve today’s health care challenges. Our employees regularly collaborate with their counterparts in the U.S. and Europe to help solve problems and share best practices. Also, to provide choice and flexibility to our clinicians, we offer multiple delivery systems of the same products wherever possible – in vials, freeflex® IV bags and Simplist® prefilled syringes.
- MORE Agility – Our world is increasingly volatile and unpredictable, so our ability to pivot is key. To be nimbler, we have incorporated numerous technological innovations to improve our speed and efficiencies. We also plan for potential disruptions so that we can maintain our agility – for example by increasing our safety stock targets for flagship products and leveraging our global network for sourcing API and components. A credit to this, during hurricanes in the Caribbean, we were able to deliver when others could not. And during the recent pandemic, our Fresenius Kabi plants were able to quickly transition to make diluent for America’s vaccines. In addition, our national distribution centers allow us to be more agile in delivering our products to our customers. Our national logistics network operates 24/7 and can deliver life-saving medicines to any hospital in the continental U.S. within 48 hours.
- MORE Dedication – Finally, the most important factor in manufacturing essential medicines is people. Our “caring for life” culture, led by strong and engaged leadership, helps connect our highly skilled employees to the patients we serve. Employees understand that we are in the business of “saving lives, one dose at a time.” Caring for life also means we cultivate transparency and collaboration with customers, cross-industry colleagues, GPOs and government agencies. We serve on the board of several forward-thinking industry organizations including the End Drug Shortages Alliance and the Association for Accessible Medicines. We believe collaboration – working across all members of the pharmaceutical supply chain – is one of the best ways to address the global issue of medication shortages. When all parties, from API developers to manufacturers to wholesalers to regulators to customers, are dedicated to solving these problems together – only then will we be able to drive change.
We know your team is working hard every day to assure you have essential, life-saving medications available for patients. At Fresenius Kabi, we are committed to doing all we can to be your reliable, trusted partner – including expanding our pharmaceutical production facilities in America and building more resilience in our supply chain – so we can bring you the quality essential medicines patients depend on.
1 The New York Times, May 17, 2023.
2 2022 data on file.
3 Following research from Harvard University, McKinsey and Gartner.
